1. The products under development are as follows:
(1) ES135 protein drug
ES135 is a recombinant human acid fibroblast growth factor (rhFGF1) with 135 amino acids. Its indication for spinal cord injury has been investigated in approximately total 300 patients in phase 1, 2 and 3 clinical trials, respectively. Currently, it is being tested in an exploratory clinical trial in patients with carpal tunnel syndrome for new indication of peripheral nerve injury.
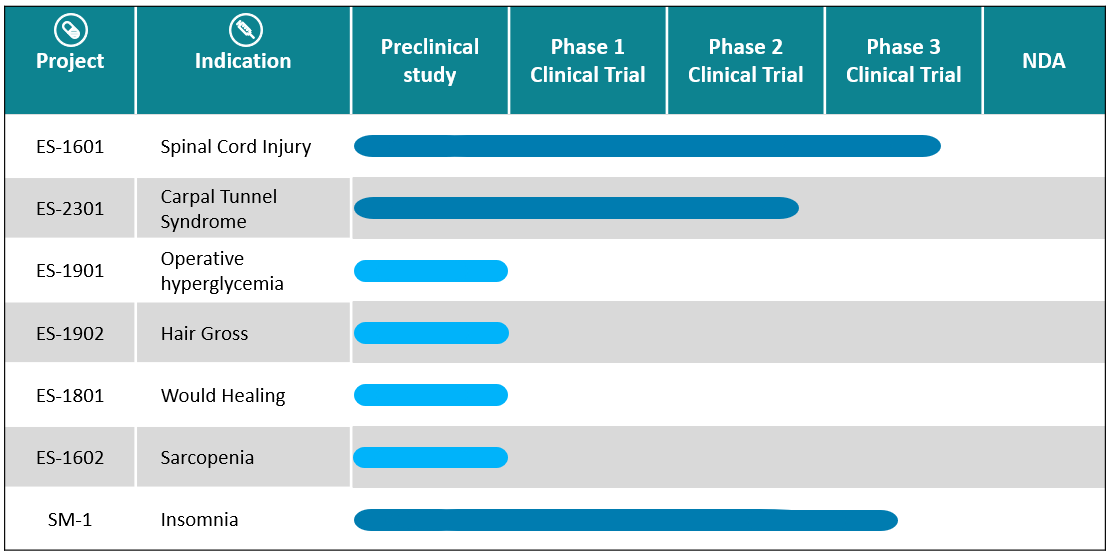
(2) SM-1 : a 3-in-1 combination sleeping aid
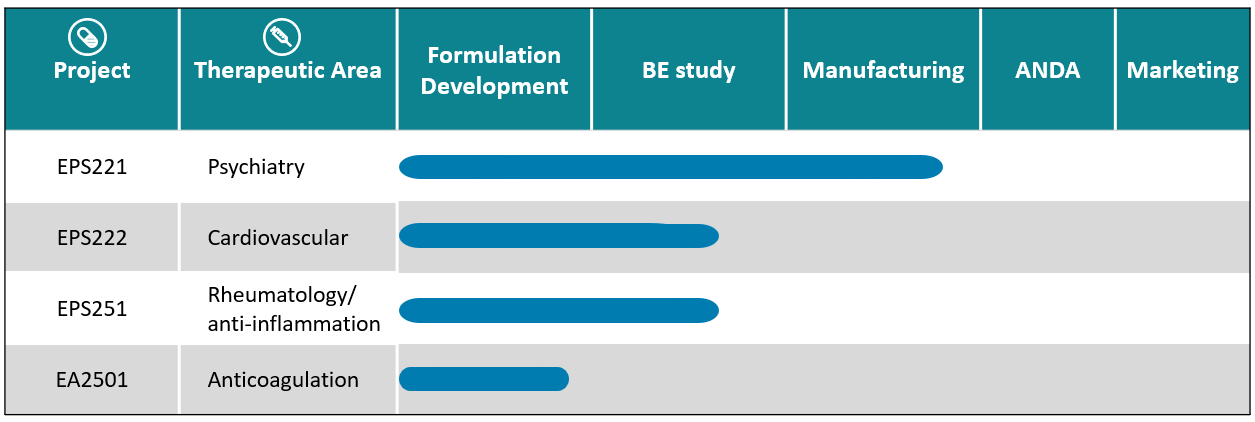
(3) EPS221 small molecule drug: a selective serotonin reuptake inhibitors (SSRIs)
(4) EPS222 small molecule drug: a calcium channel blocker for cardiovascular disease.
(5) EPS251 small molecule drug for rheumatology/anti-inflammation.
(6) EA2501 small molecule drug for anticoagulation.
(3) EPS221 small molecule drug: a selective serotonin reuptake inhibitors (SSRIs)
(4) EPS222 small molecule drug: a calcium channel blocker for cardiovascular disease.
(5) EPS251 small molecule drug for rheumatology/anti-inflammation.
(6) EA2501 small molecule drug for anticoagulation.
2. New product/Service in preparation
(1) Licensing in approved medicines and medical device
(2) Co-develop 505(b)2 new drugs with Pharmaceutical Partner(s) to expand R&D program
